 Your best protection against COVID-19 is getting one of the safe, free, and highly effective vaccines that are widely available around Arizona. Vaccination helps prevent severe illness, hospitalization, and death from COVID-19 while helping preserve healthcare capacity for others who need it.
Your best protection against COVID-19 is getting one of the safe, free, and highly effective vaccines that are widely available around Arizona. Vaccination helps prevent severe illness, hospitalization, and death from COVID-19 while helping preserve healthcare capacity for others who need it.
Complementing the state’s push for vaccination are monoclonal antibody treatments approved for emergency use by the U.S. Food and Drug Administration (FDA). Used early, monoclonal antibodies provide COVID-19 patients with a boost that can help keep COVID-19 from putting them in the hospital — or worse.
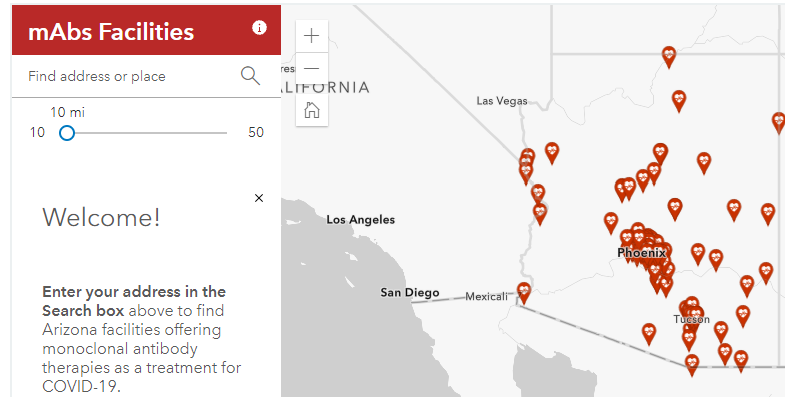
ADHS has made it easier to locate a facility offering monoclonal antibody treatment with a new resource, available in English and Spanish, providing information on eligibility and an interactive map helping patients find facilities near them. The patient resources are linked from azdhs.gov/mabs, a site established to help healthcare providers and facilities wanting to provide monoclonal antibody treatments.
Early evidence suggests that treatment with monoclonal antibodies (abbreviated as mAbs) can reduce the amount of the virus that causes COVID-19, otherwise known as viral load, in a person’s body. A lower viral load can mean milder symptoms and can reduce the chances of severe COVID-19 symptoms. That makes this treatment especially helpful to people at high risk for severe complications of COVID-19.
The criteria for monoclonal antibody treatment includes being age 12 or older with a positive COVID-19 test, having mild to moderate symptoms, being within 10 days of the start of symptoms, and being at high risk for more serious symptoms (examples: chronic lung diseases, sickle cell disease, pregnancy, and being 65 and older).
I hope you’ll check out this helpful resource so you or someone you know can act quickly to get monoclonal antibody treatment should you contract COVID-19.